Excerpts from the FDA’s clinical review of Pfizer’s Studies of Viagra and from the medication’s package insert.
"The intent was to randomize 375 male subjects > 18 with erectile dysfunction of >6 months duration, and in a heterosexual relationship for >6 months. Subjects were excluded for (1) anatomical deformities such as severe penile fibrosis, (2) spinal cord injury, (3) regular use of nitrates, estrogens, anti-androgens, anticoagulants, or psychotropic drugs….(5) stroke or myocardial infarction within 6 months…(10) suspected sexually transmitted disease…(13) alcohol or drug dependence…."
"Clinical Studies
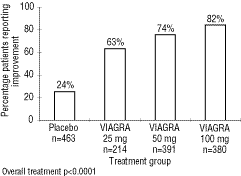
In clinical studies, VIAGRA was assessed for its effect on the ability of men with erectile dysfunction (ED) to engage in sexual activity and in many cases specifically on the ability to achieve and maintain an erection sufficient for satisfactory sexual activity. VIAGRA was evaluated primarily at doses of 25 mg, 50 mg, and 100 mg in 21 randomized, double-blind, placebo-controlled trials of up to six months in duration, using a variety of study designs (fixed dose, titration, parallel, crossover). VIAGRA was administered to more than 3,000 patients aged 19 to 87 years, with ED of various etiologies (organic, psychogenic, mixed) with a mean duration of five years. VIAGRA demonstrated statistically significant improvement compared to placebo in all 21 studies."
A graphic from the package insert:
